|
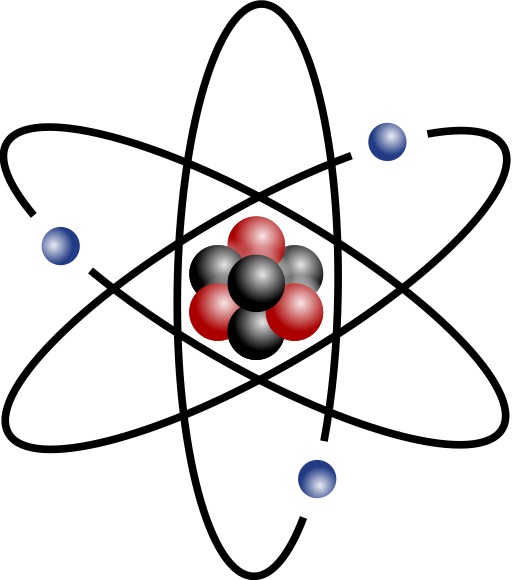
The basic structure of an atom comprises a small central
nucleus consisting of protons and neutrons (nucleons)
surrounded by electrons.
Isotopes are atoms of the same element that have different
mass numbers. (chemically identical, physically different)
Nucleus is held together by the Strong Force
- Color force holds quarks together creating baryons
- Nuclear force holds baryons together creating atomic nuclei
Lectures
Videos
Home page /
Stage 1 home page /
Nuclear home page
|