|
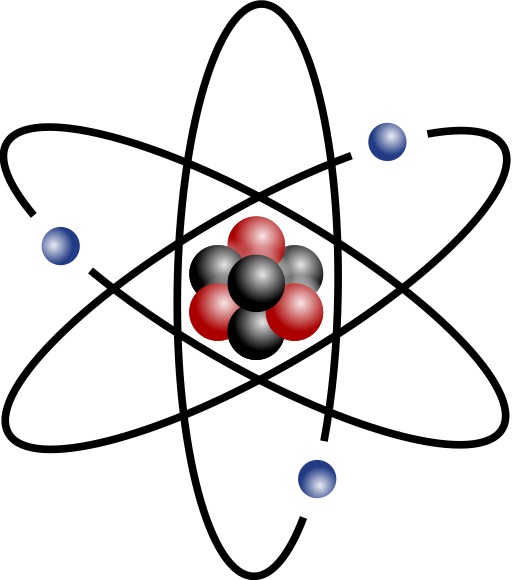
- The basic structure of an atom comprises a small central
nucleus consisting of protons and neutrons (nucleons)
surrounded by electrons.
- Isotopes are atoms of the same element that have different
mass numbers. (chemically identical, physically different)
- Nucleus is held together by the Strong Force
- Color force holds quarks together creating baryons
- Nuclear force holds baryons together creating atomic nuclei
- Unstable nuclei will undergo radioactive decay in which particles
and/or electromagnetic radiation are emitted.
- In alpha decay, an unstable nucleus emits an alpha particle
- In beta minus decay, an unstable nucleus emits an electron
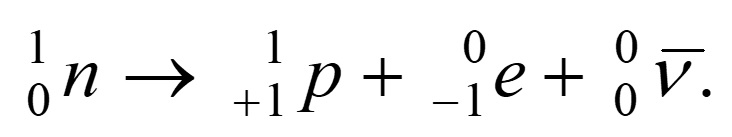
- In beta plus decay, an unstable nucleus emits a positron
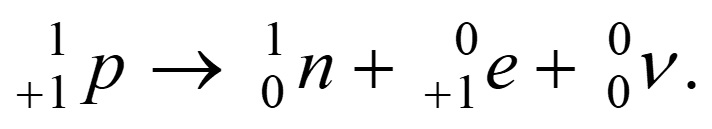
- The number of radioactive nuclei in a sample of a given isotope decreases exponentially with time.
- Half-life is the time required for half of the radioactive nuclei in a sample to decay.
- The half-life of radioactive nuclei is independent of both the physical state and the chemical state of the material.
- The activity of a radioactive substance is the number of radioactive nuclei that decay per unit time.
- The range of products of nuclear decay, some with long half-lives, means that nuclear waste must be stored for long periods.
- Nuclear fission can be induced in some heavy nuclei by
the capture of a neutron.
- The nucleus splits into two nuclei and several neutrons.
- The total mass of the reactants in a fission reaction is
greater than that of the products, releasing energy given by
E=mc2
- Chain reactions occur due to release of multiple neutrons in
nuclear fission
- Nuclear fusion is the process in which two nuclei combine into
a single nucleus.
|